While PPE and sanitation procedures have long been a part of food and beverage production, the COVID-19 pandemic caused processors to reevaluate how to protect workers without sacrificing food safety. Photo courtesy of Getty Images/Traimak_Ivan
2021 marked the anniversary of two significant events: one year of the COVID-19 pandemic and 10 years since President Obama signed the Food Safety Modernization Act into law. So, how have these two issues really affected the food industry and their food safety management systems?
Let’s first look at COVID-19 and its effect on the industry. At the start of the pandemic, the recommendations to the general public and to both the food processing industry and the foodservice/food handling sector targeted both plant workers and the general public. These recommendations were very conservative and, sadly enough, really not based on a full and complete understanding of the virus itself. The food processing industry and those supporting the industry, such as food warehousing operations, transportation and suppliers to the industry plus grocery outlets, are all essential industries. Three hundred and forty million Americans and billions of people around the world have to eat, so these industries and others had to remain open.
The early recommendations from the Food and Drug Administration and other agencies emphasized the following areas (Employee Health and Food Safety Checklist for Human and Animal Food Operations During the COVID-19 Pandemic (fda.gov):
1. Maintain distancing
2. Wear masks
3. Practice good personal hygiene
4. Handwashing
5. Clean and sanitize surfaces regularly
6. Check and interview employees daily
These recommendations focused more on protecting employee health than food safety. However, at the beginning of the pandemic, there were still concerns about how the virus could be spread. Today, we know that COVID-19 is a respiratory virus but back in early 2020, scientists were concerned that it could be spread through food or through contaminated surfaces. Research has shown that the virus does not spread through food or packaging or through food contact surfaces. This link clearly states that “there is currently no evidence to support the transmission of COVID-19 associated with food or packaging.”
Adjusting to the brave new world of COVID-19 was a challenge for food processors, but not as great as it could have been. Handwashing and good personal hygiene are, or should have been, integral elements of the prerequisite programs for all food processors. Standard practice in food processing operations was and remains as follows: Wash your hands when entering a food processing facility and as needed when on the floor. These issues were and continue to be emphasized. Cleaning and sanitizing food contact surfaces and the remainder of the plant are also essential prerequisite programs. This is one element that received additional emphasis during the pandemic.
COVID-19, FSMA have widespread effects on food safety
The state of food safety
Companies that adopt mask wearing as a standard will probably take a long look at the type of masks that must be worn. If workers must wear a mask, it is imperative that they not only be comfortable, but safe. In this case, safety means worker safety. As one who must wear glasses, I know that my lenses have a tendency to fog up, especially in cold weather. Any time vision is compromised it poses a safety risk.
The last element is distancing. Many processors have modified operations to ensure that workers maintain proper distancing. Others have installed shields to separate workers. The use of shields is an effective means of protecting workers. Whether the shields remain when the pandemic ends also remains to be seen. Installation of shields has added one complication to plant operations, however. The shields must be cleaned and sanitized so by installing shields, processors had to expand their cleaning programs.
To minimize the potential of spreading the virus, mask wearing has been adopted throughout the industry. This was standard for some operations prior to the onset of the pandemic. Whether this continues as a standard when the pandemic ends remains to be seen, but it is very likely that many companies will do so, especially in the meat processing industry.
FSMA at 10
Let’s take a look at what has gone on in the 10 years since the Food Safety Modernization Act was signed into law. First and foremost, the program was not HARPC or Hazard Analysis Risk-based Preventive Controls or HACCP on steroids. The use of the term HARPC was unfortunate in that many processors felt that they would be asked to adopt a whole new food safety management system, which was not the case. The law and the regulations that have been enacted to ensure its enforcement are really traditional HACCP with greater emphasis on certain areas such as risk assessment, process validation and supplier controls. And, the regulation also mandates that these programs be documented and that they have been properly implemented.
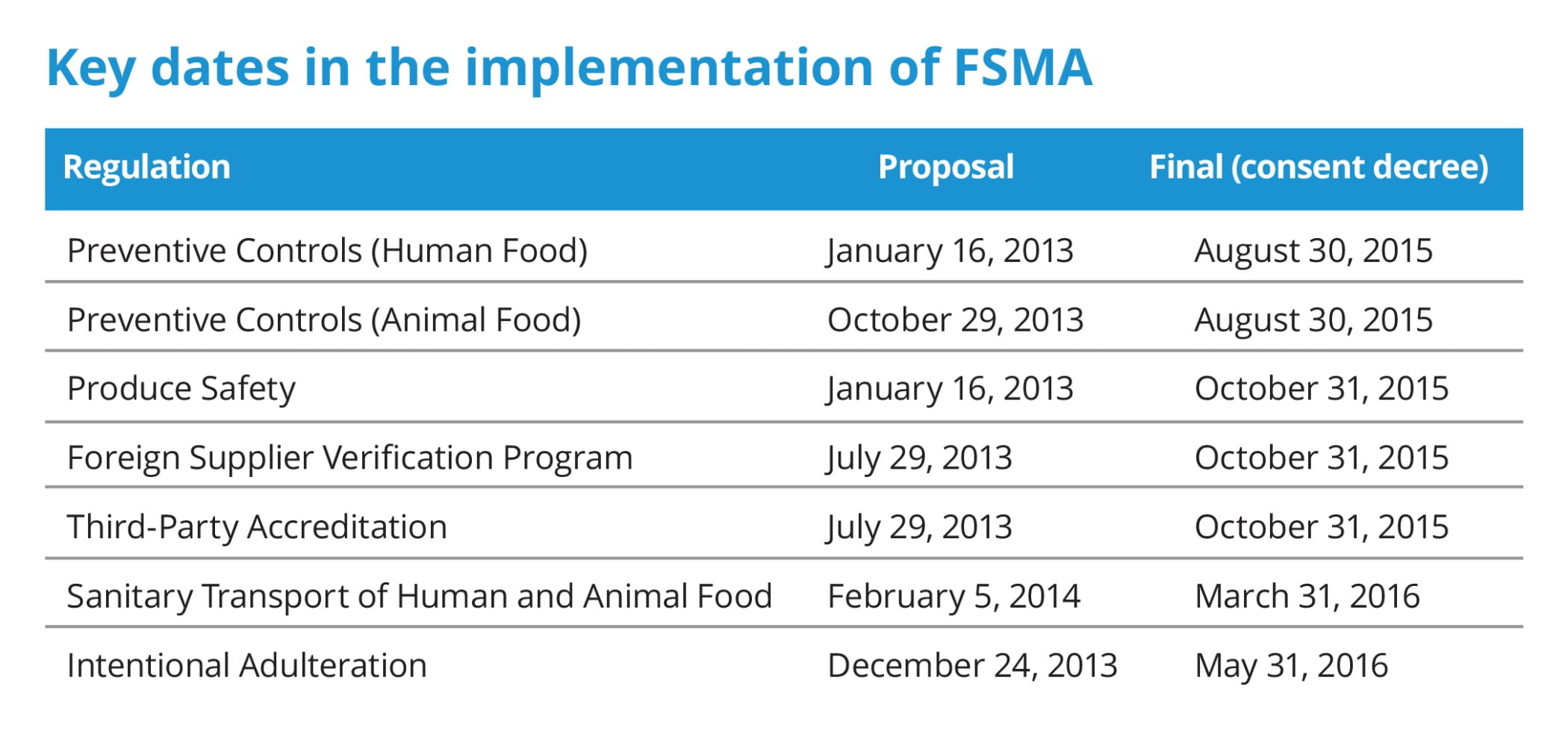
Part of the challenge to the industry was that even though the FSMA was signed into law in January 2011, it took between 4½ and 5½ years to finalize the regulations needed to define and enforce the law (See Figure 1). This delay in developing the regulations also created some confusion, since there were those offering their services to the industry to assist them with FSMA compliance. How can one offer such services without knowing what would be in the regulations?
There are different tools for assessing risk currently in use. Perhaps the most common is a system in which the company assigns a value to likelihood of occurrence and severity, and multiples them together to get a risk rating. The higher the final value, the greater the risk.