Risk-based Sanitation - Part 2
Having a plan and taking action on a continual basis helps keep uninvited squatter bacteria out of your facility.
Develop a
to Sanitation
a Risk-based Approach
HACCP, HARPC or Both
HACCP has been around for some time, but HARPC (hazard analysis and risk-based preventive controls) came out of FSMA. What is the better approach with cleaning and sanitation?
“The answer depends on the regulatory and/or customer requirements in place,” says Stokes. “For most processors, this choice is determined by the product that is being made. Regulations may require HACCP or HARPC approach.”
Rachel McGinness, lead chemical engineer at Ecolab, completes a titration via test kit in a small dairy plant to check CIP effectiveness. Image courtesy of Ecolab
by Wayne Labs, Senior Contributing Technical Editor

A primary packaging system for candy operates in an enclosure to keep it shielded from outside air. Washing down equipment in an operation like this could do more harm than good. Image courtesy of Imagemakers Inc.
If a process has a relatively narrow product exposure window, and if the sanitation is controlled through very repeatable means like CIP or closely inspected after every cleaning, then a manufacturer may not see as many benefits in the HARPC philosophy, adds Stokes. “But I do see advantages of the HARPC approach to systems that have a wide exposure window or limited treatment to address potential hazards. In this case, HARPC offers an opportunity to really explore the risks along the entire chain from supply to consumer.”
“If HACCP is used as intended, I think you can create an effective sanitation and environmental monitoring program without adding HARPC,” says Richards. The first step to HACCP is creating the cross functional team—people from all different positions in the facility so you have extra eyes on the process. Once you have a team in place, then you can start to discuss the hazards and how you plan to control them—whether that’s through control points, cleaning or personnel training. To create an effective cleaning program, you’ll need to collect data to prove that your cleaning procedures are effective. This means swabbing the equipment and facility before and after cleaning to understand if you’re getting the equipment clean and free from microbial growth. You can then use that data to track and trend when you do have a presumptive positive in a specific area.
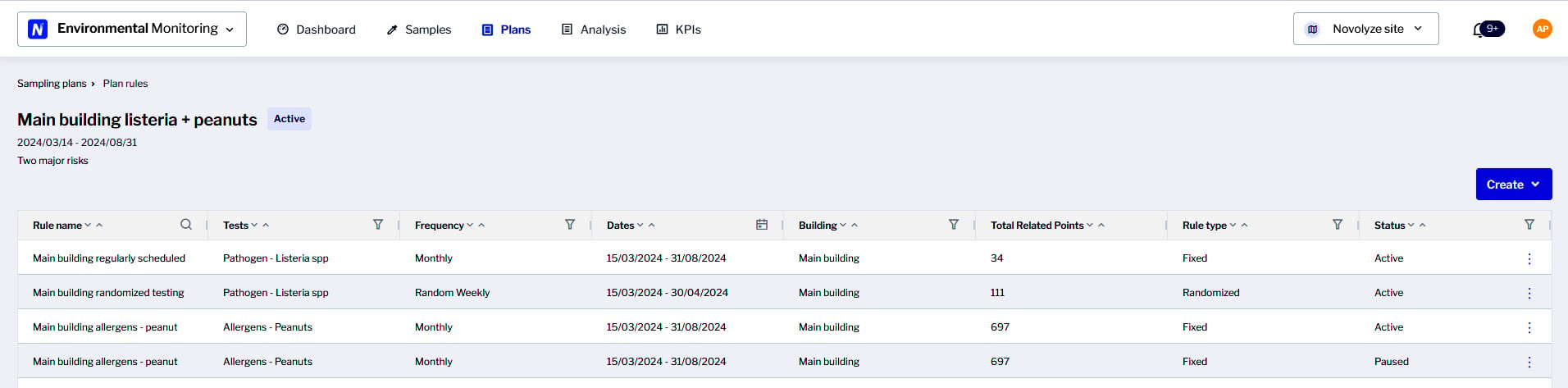
A good way to organize and collect data from different points in the plant is through the use of an environmental monitoring system. Image courtesy of Novolyze
“Integrating the principles of both HACCP and HARPC would be a better approach as it recognizes the interconnectedness of product and environmental risks and addresses them together,” says Chua. “This also provides clear direction to all stakeholders at all stages of the project’s life cycle.”
A Risk-based Approach to Sanitation/Cleaning
How can processors develop an effective approach to sanitation/cleaning and hygiene that prioritizes the right areas of the plant and/or equipment to be cleaned and meet regulatory rules?
“Identify the risks and their severity within each area of the facility,” says Gray’s Basham. Develop a schedule of cleaning based on the severity of the risks within that area, cleaning the areas that pose a greater threat more often or with more stringent practices than those that do not. Understanding the types of pathogens that could be present in different areas of the facility and the best approach to their mitigation is paramount. Using a wet cleaning method in a dry area will most times do more harm than good.
It all comes down to effective monitoring and better use of sanitation zoning, says Ecolab’s Stokes. You can set up your cleaning methods and tools as rigorously as you want, but without attentive monitoring and meaningful adaptation to data they’re not going to make the impact they’re capable of making. It’s important to begin with a thorough understanding of what you’re monitoring for and where you can find that information, and the results of that data collection will feed back into a formalized review process that helps you continuously reestablish your risk profile.

This raw meat processing area is completely cut off from the cooked/RTE side of the plant with separate employee entrances for each side, preventing bacteria from entering the finished product areas. Image courtesy of Ross Van Pelt, RVP Photography
“My advice to customers is this: Treat regulatory rules as a minimum bar,” adds Stokes. “Develop an effective sanitation program that addresses the full spectrum of your sanitation-related risk. Your company is the expert when it comes to what you make and how you make it, so direct that deep knowledge toward creating a superior sanitation program (complete with employee training and effective tools) that thoroughly meets the site-specific requirements for keeping your product safe. Then, review regulatory requirements and other sanitation-related standards to ensure that your program hasn’t left any gaps covered by specific regulation.”
And don’t forget metrics. “Identifying measurable metrics against risk exposure and probabilities can help food processors develop an effective risk-based approach to sanitation and cleaning,” says Chua. “Metrics allow for focused targets versus vague ideals, therefore providing a baseline and point of verification for effectivity.” An environmental monitoring program can establish procedures and methods in cleaning, sampling, analyzing and trending.
“Understanding risk requires strong analysis and data supporting risk identification,” says Ndiaye. “Data visualization tools, such as those offered by Novolyze Environmental Monitoring and Sanitation Complete, are necessary to spot worst case areas and equipment.”
What Food Processors Need in Their Food Safety Plans
• Food processors could benefit from more fully integrated standards. Standards may be PDF files that are stored but not easily available to support day-to-day activities.
• Food processors could benefit from increased ability to manage task accountability and ways to drive accountability and ownership.
• In cases where the standard lifecycle and training are poorly handled and not scalable, relying on shadowing as training can lead to a culture that is stable, but potentially missing out on innovation.
• Food processors could benefit from greater integration of CAPA (corrective and preventive action) and continuous improvement in their sanitation campaign management. However, data may not be visible enough and/or not usable for CAPA and continuous improvement.
• Food processors could benefit from a greater interconnection between environmental monitoring, cleaning and sanitation, and process control. Otherwise, when failures appear and new risks emerge, there may not be the agility to adapt. A solution to this dilemma is a food safety/quality platform that covers all of these areas, allowing for food safety, quality assurance and operational efficiency.
—Moussa Ndiaye, Product Manager, Novolyze
Resident Bacteria: Eradicate or Demolish the Building?
Sometimes resident bacteria so permeate a facility that it’s impossible to get rid of the bugs without razing the structure. I can remember one or two such cases. But it doesn’t have to be this way.
“Razing the entire facility or a ‘scorched earth’ approach, although an option, should be a last resort,” says Gresham Smith’s Chua. “There is still hope in eradicating the bacteria if there is a clear methodology to isolate the incidence and understand how it occurred. In most cases, this clear path is through an environmental monitoring program with sampling, testing and analysis.”
Think about how bacteria can become resident bacteria, says Novolyze’s Ndiaye:
• Adaptation to chemicals—bacteria can adapt to chemicals and stress
• Shielded location—bacteria accumulate in hard-to-reach areas
• Ineffective sampling plan—sampling in the wrong locations; sampling plans need to be adaptive and evolve over time
Changing chemicals regularly and efficient mapping of sampling plans can support identifying bacteria in shielded areas, adds Ndiaye.
“Applying strong sanitation principles designed to limit or remove bacteria will often remove resident organisms,” says Ecolab’s Stokes. “I’ll add that a robust understanding of microorganisms and the tools used to detect them is essential.”

Rachel McGinness, lead chemical engineer at Ecolab, pulls a sample to measure pH via portable device to verify that the chemical has sufficient strength to kill bacteria in a CIP process. Image courtesy of Ecolab
“It’s tempting in these situations to look for a ‘smoking gun’—a single source or trigger for the problem, says Stokes. But looking at it this way may miss the root of the problem. More often than not, it’s not a single source but a network of poor conditions that make resident organisms possible. I have seen many companies target the symptoms instead of attacking the root of the problem, and this approach rarely yields positive results.”
Typically, you would rely on your swab data to track where the listeria hits are showing up and do a ‘swabathon’ in adjacent areas to see where it’s going,” says Gray’s Richards.1 “Once you have a map of hotspots, you can start with a deep cleansing of those areas, while minimizing foot traffic to avoid tracking it into other areas. After you have effectively cleaned all contaminated areas, continue with another swabathon to ensure that the areas can resume production. Once production has returned to normal, the food safety team should do a corrective action report to understand how the listeria was able to form and why it wasn’t detected before it got out of hand. The cause could be due to the cleaning schedule and not being cleaned as frequently as needed, but I have found that training is usually the number one cause of listeria or salmonella outbreaks.” FE
Sources:
1 “What is a Swabathon?: Understanding the FDA Investigations,” Mérieux NutriSciences, (July 13, 2017) Website accessed 3-29-2024
“Foodborne Biological Hazards: Sampling for foodborne biological hazards, and filth– domestic and import,” FDA Compliance Program Guidance Manual, FDA (6-03-2022)


